
Vecollan® - Next-generation collagen
Collagen is an important material in modern medicine. Creavis developed an animal-origin free collagen that is safe, sustainable, and commercially scalable. The high-purity product can be reliably processed and simplifies the regulatory approval of medical devices.
Collagen is frequently used in pharmaceutical applications, in special implants, in artificial tissue, and also in cosmetic surgery. Collagen is found primarily in bones, muscles, skin, and tendons. There it provides strength and structure. When physicians use collagen in the laboratory or in surgery, they have so far resorted to animal material. It comes from cattle, for example, but also rats, jellyfish and more. This has disadvantages: The quality of animal collagen varies from batch to batch and purification to clinical grade quality is costly. In addition, even small contaminations of animal collagen can potentially cause undesirable immune reactions.
From a bioreactor, not from an animal
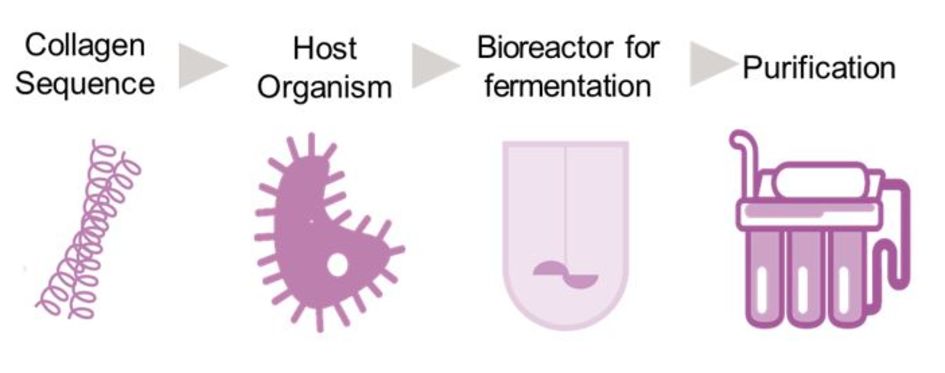
We at Creavis have developed an alternative: recombinant collagen, produced by fermentation in a bioreactor with the help of tailor-made microorganisms - without any animal starting material at all. After purification, the product not only meets strict quality requirements, but its characteristics are also more consistent than animal-derived collagen. It is also particularly similar to human collagen. It therefore interacts reliably with human cells and can be easily absorbed or remodeled by the body.
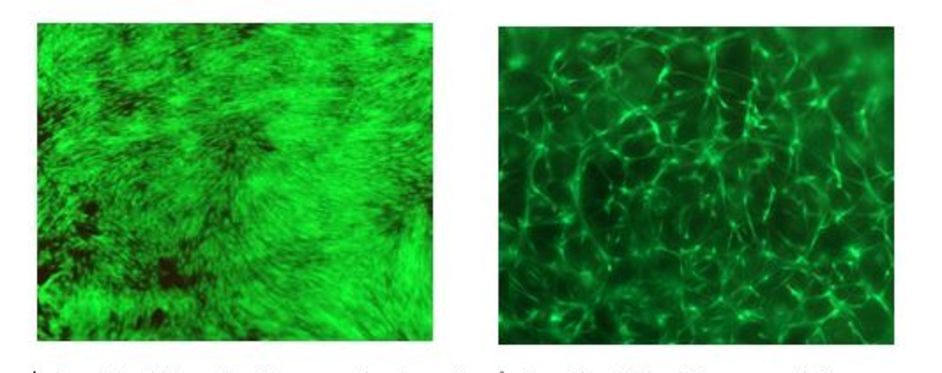
left: In-vitro 2D cell culture on hydrogel surface, good cell attachment and proliferation, human dermal fibroblasts
right: In-vitro 3D cell encapsulation, extensive cell spreading, human dermal fibroblasts
Available in any form
Our biotechnologists have developed the process into a technology platform called Vecollan®, a new system solution for the medical technology industry. The combination of both the fermentation and the purification processes makes the product particularly safe, sustainable, and scalable. In addition, Vecollan® is highly adaptable and versatile as a material. It can be processed in many forms, for example as hydrogels or sponges. This allows it to be used as a filler in plastic surgery as well as a scaffold in tissue engineering, a coating for implants or even in ink for medical 3D printing.
Opening up new markets
Vecollan® was developed at Creavis, Evonik's strategic innovation unit and business incubator, in cooperation with several of the Group's business units and research facilities in Europe, Asia, and North America. This included the biotechnology platform of the Nutrition & Care Life Science Division. The Health Care Business Line aims to increase the share of such system solutions in its portfolio to more than 50 percent by 2030. As a business incubator, Creavis facilitates the development of such new solutions, which have not previously been part of our core business.
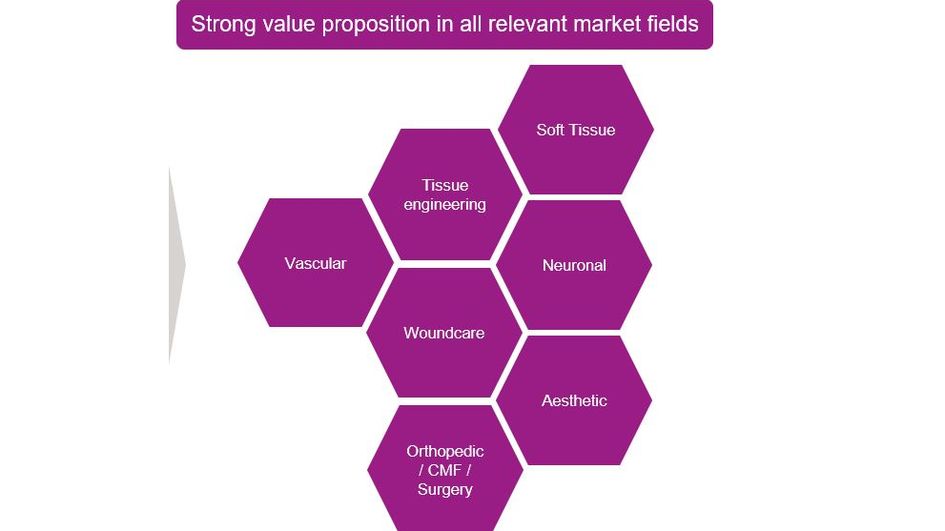
Enabling new therapies
Vecollan® is not just a valuable addition to Evonik's portfolio for the medical technology industry. Such next-generation nature-identical biomaterials are also likely to spur the development of new and safe therapies that would not have been feasible before. By developing a scalable and market-ready collagen platform, we are also helping to improve the quality of life for millions of people.
